Ring Double Bond Equivalents (RDBE) or Double Bond Equivalents (DBE) are calculated from valence values of elements contained in a formula and should tell the number of bonds - or rings. Well. This formula is a relict or even a relic from times when only a few people knew about graph theory in chemistry and had no access to fast computers and structure generators. Even worse, this formula in its original definition also produces wrong values. That is the reason modern formula generators also report an RDBE range and not a single value. A novel formalism of was devolped for that purpose.
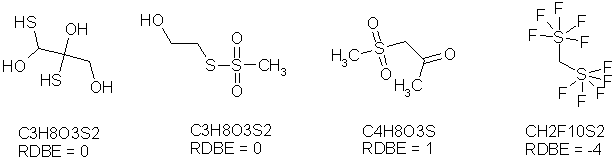
The first formula C3H8H3S2 has no rings and double bonds which OK. The second one has 2 double bonds; which should be ignored? The third formula C4H8O3S has 3 double bonds, but only a RDBE of 1. Well if one should ignore all the double bonds why calculate in the first place? And the fourth example the existing bis(pentafluorosulfur)methane CH2F10S2 has a negative ring double bond equivalent of -4. There are hundreds of such examples, especially from organic compounds containing halogenes (F,Cl,Br,I) together with sulfur, nitrogen and phosphorous.
One of the more simpler reasons why this formula is of no great help is, that complex organic mixtures contain multiple elements, which also can have different valence states. Such as sulfur and
phosphorus compounds. Now simply imagine a formula or compound which has different sulfur and phosphorus goups included (thioether or thiol v=2,
sulfoxide (v=4),
sulfone (v=6) and phosphine (v=3) and phophonates (v=5). Now this will generate a wrong result using the RDBE formula. For advanced structure elucidation processes the
RDBE or DBE values of molecular formulas are of
no great help. Correct use of LEWIS and SENIOR rules together with Molecular Isomer Generators is the
preferred way.
Example: C2H6SO2
- S (valence = 2) : 24 structural isomers
- S (valence = 4) : 95 structural isomers
- S (valence = 6) : 166 structural isomers
For natural compounds where only a few hundred halogen containing compounds exist, the ring double bond equivalents contain no negative RDBEs. For other complex matrices like crude oil, natural organic matter or hydrogen mixture exist some concepts to utilize RDBs either due to the lack of structure elucidation concepts or due to reductionist concepts which are sometimes helpful and sufficient.
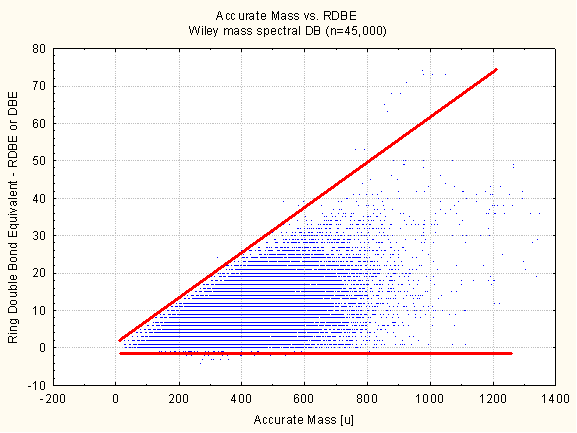
The picture accurate mass vs. RDBE from 45,000 formulas clearly shows that for every mass range the RDBE can not exceed a certain value. This is due to the well known Senior Rule (is a molecular graph possible or not) or in a simple example the formula C4H10 exist but C4H12 does not. For small molecules the RDBE has a histogram maximum at around 7 and below 1000 Da the RDBE usually does not exceed 40.
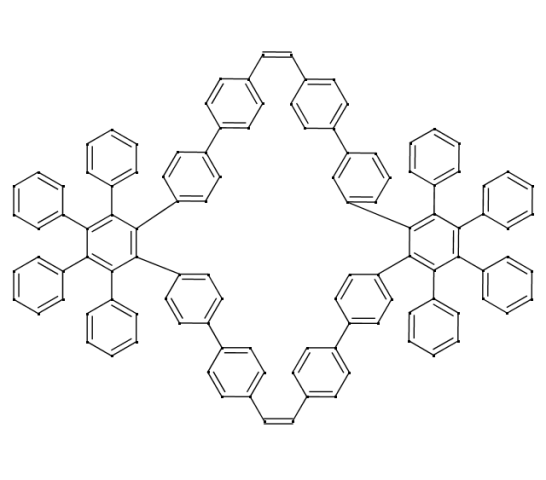
However as seen from the graphics which shows the existing molecule 1,2,3,4,23,24,25,26-octaphenyl-5,8:9,12:15,18:19,22:27,30:31,34:37,40:41,44-Octaethenodibenzo[a,u]cyclotetracontene; C112H76, RDBE = 75, Mass = 1420.59467u [PDF] one can not exclude RDBE values, because with increasing molecular masses the RDBEs also increase and are limited by the SENIOR rules and the Seven Golden Rules anyway.
Additional links:
- Chemical Graph Theory: Introduction and Fundamentals
- History and Progress of the Generation of Structural Formulae in Chemistry and its Applications
- Analogous odd-even parities in mathematics and chemistry
- A Novel Formalism To Characterize the Degree of Unsaturation of Organic Molecules
- Characterization of hydrocarbon systems by DBE concept
- From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter
- The even-electron rule in electrospray mass spectra of pesticides
- Even-electron ions: a systematic study of the neutral species lost in the dissociation of quasi-molecular ions